

- Chemical equation balancer with phases code#
- Chemical equation balancer with phases plus#
Syntax guide Feature & demoįoo 5+ + Bar 3â â FooBar 2 + FooBar â
Chemical equation balancer with phases code#
The source TypeScript code and compiled JavaScript code are available for viewing. This program was hand-written in JavaScript in year 2011, received minor feature updates and clarifications and refactorings throughout the years, and was ported to TypeScript in 2018. Because the program is entirely client-side JavaScript code, this web page can be saved and used offline. The algorithm used is Gauss-Jordan elimination, slightly modified to operate using only integer coefficients (not fractions). The program calculates the coefficients to balance your given chemical equation. This is an easy-to-use, no-nonsense chemical equation balancer.
One mole of methane molecules and 2 moles of oxygen molecules react to yield 1 mole of carbon dioxide molecules and 2 moles of water molecules.Chemical equation balancer (JavaScript) Program Input:. One dozen methane molecules and two dozen oxygen molecules react to yield one dozen carbon dioxide molecules and two dozen water molecules. 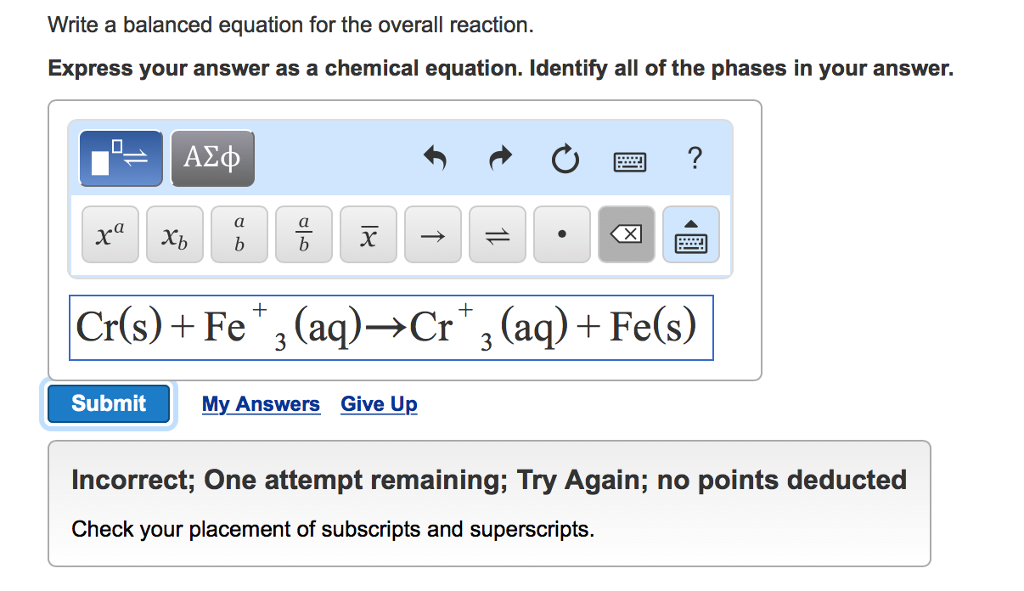
One methane molecule and two oxygen molecules react to yield one carbon dioxide molecule and two water molecules.Likewise, these coefficients may be interpreted with regard to any amount (number) unit, and so this equation may be correctly read in many ways, including:
/hand-writing-on-blackboard-a0022-000119-5907b22d3df78c92831484b9.jpg)
This ratio is satisfied if the numbers of these molecules are, respectively, 1-2-1-2, or 2-4-2-4, or 3-6-3-6, and so on ( (Figure)). Methane and oxygen react to yield carbon dioxide and water in a 1:2:1:2 ratio. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example.
The relative numbers of reactant and product species are represented by coefficients (numbers placed immediately to the left of each formula). Chemical equation balancer with phases plus#
Plus signs (+) separate individual reactant and product formulas, and an arrow separates the reactant and product (left and right) sides of the equation.The substances generated by the reaction are called products, and their formulas are placed on the right side of the equation.
 The substances undergoing reaction are called reactants, and their formulas are placed on the left side of the equation. This example illustrates the fundamental aspects of any chemical equation: The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). The chemical equation representing this process is provided in the upper half of (Figure), with space-filling molecular models shown in the lower half of the figure. Consider as an example the reaction between one methane molecule (CH 4) and two diatomic oxygen molecules (O 2) to produce one carbon dioxide molecule (CO 2) and two water molecules (H 2O). Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Write and balance chemical equations in molecular, total ionic, and net ionic formats.Īn earlier chapter of this text introduced the use of element symbols to represent individual atoms. Derive chemical equations from narrative descriptions of chemical reactions. By the end of this section, you will be able to:
The substances undergoing reaction are called reactants, and their formulas are placed on the left side of the equation. This example illustrates the fundamental aspects of any chemical equation: The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). The chemical equation representing this process is provided in the upper half of (Figure), with space-filling molecular models shown in the lower half of the figure. Consider as an example the reaction between one methane molecule (CH 4) and two diatomic oxygen molecules (O 2) to produce one carbon dioxide molecule (CO 2) and two water molecules (H 2O). Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Write and balance chemical equations in molecular, total ionic, and net ionic formats.Īn earlier chapter of this text introduced the use of element symbols to represent individual atoms. Derive chemical equations from narrative descriptions of chemical reactions. By the end of this section, you will be able to:




/hand-writing-on-blackboard-a0022-000119-5907b22d3df78c92831484b9.jpg)



 0 kommentar(er)
0 kommentar(er)
